FDA Food Labeling Requirements: Basic Rules and Information
What are the FDA Food Labeling Requirements?
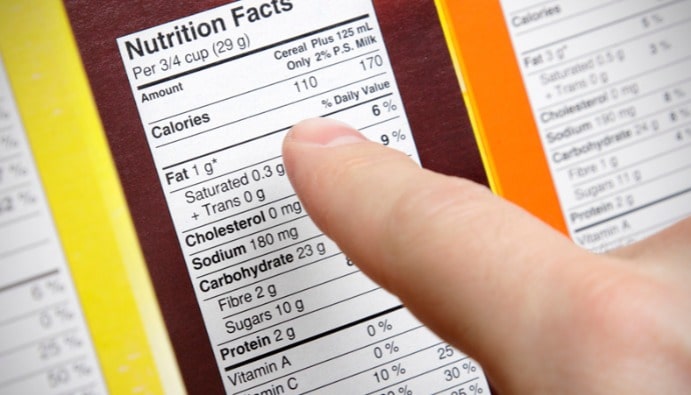
FDA Food Labeling Requirements
Food labeling is the easiest way for consumers to have information about product content. The necessary conditions and limits are specified within the scope of food labeling prepared by the FDA (US Food and Drug Administration). There are differences in labeling criteria according to the Turkish Food Codex.
Criteria set by the FDA In order to meet the requirements under the labeling rules, testing and analysis procedures must be carried out on foods.
Information about nutritional values in food labeling published by the FDA is as follows in general terms:
- Calories, Total Calories: The energy content for a serving can also be expressed in kilojoule units, with the calorie content statement immediately followed in parentheses. Calories can be calculated using the “Atwater Method”.
- Total Lipid: Total lipid refers to the total number of grams of fat in a serving expressed as triglycerides, defined as total lipid fatty acids, where fatty acids are aliphatic carboxylic acids consisting of an alkyl chain. If a serving contains less than 0.5 grams, the content can be expressed as zero.
- Saturated Fat: The number of grams of saturated fat in a serving, defined as the sum of all fatty acids that do not contain double bonds, excluding the label declaration of saturated fat content information.
- Trans Fat: Refers to the number of grams of trans fat in a serving, defined as the sum of all unsaturated fatty acids in a serving that contain one or more isolated (i.e., unconjugated) double bonds.
- Cholesterol: Label declaration of cholesterol information is not required for products containing less than 2 milligrams of cholesterol per serving.
- Sodium : Can be expressed as zero if less than 5 mg per serving.
- Total Carbohydrate: Except that the number of grams of total carbohydrate in a serving is expressed to the nearest gram, if a serving contains less than 1 gram, “Alternatively, 1 gram” or “less than 1 gram” may be used, or the content may be expressed as zero if the serving contains less than 0.5 grams.
- Dietary Fiber: if a serving contains less than 1 gram, a dietary fiber declaration is not required, or alternatively “contains less than 1 gram” or “less than 1 gram” may be used.
- Total Sugar: A label declaration of sugar content is not required for products containing less than 1 gram of sugar in one serving if no claims have been made about sweeteners, sugars or sugar-sweetened alcohol. If a serving contains less than 1 gram, it is expressed to the nearest gram.
- Added Sugars: Label declaration of added sugars is not required for products containing less than 1 gram of added sugars per serving, unless specifically requested.
- Protein: The number of grams of protein in a serving, expressed to the nearest gram, may be expressed as “contains less than 1 gram” if a serving contains less than 1 gram, or alternatively 1 gram may be used and the content expressed as zero if the serving contains less than 0.5 grams.
- Vitamins and Minerals: Vitamins and minerals such as vitamin D, calcium, iron, potassium, vitamin A, vitamin C, vitamin E, vitamin K, thiamine, riboflavin, niacin, vitamin B6, folate, vitamin B12, biotin, pantothenic acid, phosphorus, iodine, magnesium, zinc, selenium, copper, manganese, chromium, molybdenum, chloride, choline and recommended daily intakes for “adults and children, infants, children aged 1-3 years, pregnant and lactating women”.
Nanolab Laboratories Group continues to provide services within the scope of Food Analysis.
Contact us for more information.
You can follow us on LinkedIn for up-to-date news and posts about our services.
Follow our Instagram account to be informed about our latest blog posts.

