
BLOG
KATEGORİDEKİ DİĞER YAZILAR
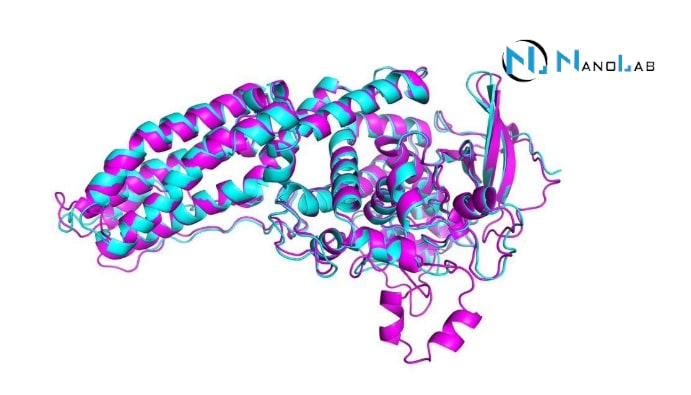
Proteins are one of the fundamental building blocks of biological systems and must have the correct three-dimensional structure to fulfill their function. Understanding protein structure and dynamics helps to better understand biological processes. Nuclear Magnetic Resonance (NMR) Spectroscopy is a powerful tool to analyze these structures and study the functions of proteins. In the structural analysis of proteins, their interactions and dynamic processes, NMR plays an important role in biochemistry and molecular biology.
Nuclear Magnetic Resonance (NMR) is a technique that measures the response of atomic nuclei to changes in their energy levels in a magnetic field. NMR is used to study interactions at the atomic level, bonding and the dynamics of molecules. Biomolecules such as proteins are studied by analyzing the signals arising from the interactions of atomic nuclei with a magnetic field.
To understand the structure of proteins, NMR provides data at atomic resolution and is used to resolve the three-dimensional structure of proteins. This allows us to better understand the functional mechanisms and interactions of proteins.
NMR has a wide range of applications in protein research. Here are some important uses of NMR spectroscopy in protein analysis:
NMR is widely used to resolve the structure of proteins in solution. Compared to X-ray crystallography, NMR may be more suitable for determining the structure of proteins in solution because proteins are naturally present in solution. This method is particularly useful for proteins that have difficulty taking crystalline form.
NMR is a powerful tool for studying the interactions of proteins with nucleic acids such as DNA or RNA. These interactions play critical roles in cellular processes and NMR helps us understand the mechanism of such interactions. These analyses are used to understand the functions of proteins that control genetic expression.
NMR is also used to study protein-ligand interactions. Small molecules that bind with a protein are critical in the drug development process. NMR can be used to determine binding sites and binding strengths. Furthermore, this method is highly effective for understanding the binding conformations and dynamics of ligands.
Dynamic NMR is used to understand the dynamic behavior of proteins. This allows to study the transitions of proteins between different structural states. Dynamic NMR helps to understand the functional states and folding processes of proteins.
NMR can also study modifications of proteins (phosphorylation, acetylation, glycosylation, etc.). Such modifications can significantly affect the functions and interactions of proteins. NMR provides information about the biological activity of proteins by determining where these modifications occur.
NMR can be used to resolve the structures of complexes formed by more than one biomolecule. Structural analysis of complexes formed with proteins, RNA/DNA and other biomolecules can reveal how these interactions occur and what mechanisms are at work.
Nanolab Laboratories Group continues to provide services within the scope of Food Analysis.
Contact us for more information.
You can follow us on LinkedIn for up-to-date news and posts about our services.
Follow our Instagram account to be informed about our latest blog posts.